Four Cities. Four Topics
Click the city tabs on the left to learn specific details of each location's event.
Munich, Germany
Predictive In Vivo Humanized Models for Immunotherapy Drug Development
At this event, we will discuss the evolution of immune system humanized mouse models and their use in the preclinical development of immunotherapies. The symposium is the ideal forum for everyone interested in generating translational data for developing therapies targeting the human immune system.
Zürich, Switzerland
Translational Models for Antibody Drug Development
The seminars will focus on the role of genetically and immune system-humanized models in the antibody drug development pipeline. Antibody developers will hear how leaders in the field use humanized models to predict and optimize the clinical performance of their drug candidate.
Paris, France
Preclinical Evaluation of Advanced Cell-Based Therapies Using In Vivo Humanized Models
We will discuss the main features of well-characterized and novel immune system humanized mouse models and their application to evaluate the preclinical efficacy and safety of cell-based cancer therapies. This event is an opportunity for developers of cell therapies to discuss how to utilize these new humanized in vivo systems to generate relevant translational data supporting their development programs.
London, UK
Principles and Applications of Humanized In Vivo Models to Study the Immune System and Evaluate Immunotherapies
We will introduce immune system humanized mice, their use to explore different characteristics of the human immune system, and their utility in testing the efficacy and safety of immunomodulatory drugs. By participating in this symposium, researchers will learn why humanized mice are quickly becoming the gold standard tools in the preclinical development of immunomodulatory therapies.
Predictive In Vivo Humanized Models for Immunotherapy Drug Development
Le Méridien Munich
Bayerstraße 41
80335 München
12 June 2023
9:00 - Registration Open
10:30 - Opening Statements
10:40 - An Introduction to the Biology of Humanized Mice
Adriano Flora Ph.D. - The Jackson Laboratory
11:15 - Immunobiology of Human Immune Engrafted Platforms for Preclinical Drug Discovery
Brian Soper Ph.D. - The Jackson Laboratory
11:50 - A Humanized Mouse Model to Study Activity of Cytotoxic and Immunomodulatory Human IgG
Anja Lux Ph.D. - FAU Erlangen
12:30 - Lunch
13:30 - Use of Humanized Mouse Models for Discovery of Novel Immunotherapies for the Treatment of Multiple Myeloma
Carina Hage Ph.D. - Roche
14:10 - Humanized Mice to Test the Safety of Immunotherapies
James Keck Ph.D. - The Jackson Laboratory
14:50 - Closing Remarks
Speakers

Anja Lux, Ph.D.
Friedrich-Alexander-University Erlangen, Germany
Anja Lux is a professor for Integrated Immunology and research group leader at Friedrich-Alexander-University Erlangen-Nürnberg. The main research interests of the Lux lab are on immunoglobulin G antibodies and their effector functions induced by cellular Fc receptors and the complement system. They have a long standing expertise in using humanized mouse models for studies on IgG activity in terms of cytotoxicity and immunomodulation.

Carina Hage, Ph.D.
Scientist and Group Lead, Roche Diagnostics GmbH
Carina Hage, studied Molecular Biomedicine at the WWU Muenster and obtained a Ph.D. in Oncology. Since 2019 she works as scientist and group lead in the Discovery Oncology at Roche Diagnostics in Penzberg (Germany). She is working with humanized models to assess the mode of action and efficacy of novel cancer immunotherapy drugs and investigates the translatability of preclinical models in drug development.

Brian Soper, Ph.D.
Senior Scientific Engagement Manager - The Jackson Laboratory
Dr. Soper has worked at The Jackson Laboratory for more than 26 years. He has conducted research on hematopoietic stem cell biology, bone marrow transplantation, immune tolerance, and treatment strategies for a mouse model of human enzyme deficiency. He now works as Senior Scientific Engagement Manager providing highly technical scientific educational resources to the scientific and drug development community. Brian's expertise includes the immuno-biology of humanized mice following human PBMC or hematopoietic stem cell transplantation, and their use to study human immune modulation and immuno-oncology when co-transplanted with human tumors.

James Keck, Ph.D.
Senior Director for Innovation and Product Development - The Jackson Laboratory
Dr. Keck is the recent recipient of JAX's Presidential Innovation Fellow Award. In this role, he is responsible for developing and driving cutting-edge immuno-oncology and autoimmune platforms and services to empower preclinical drug developers across the globe, as well as overseeing internal and external collaborations that may lead to new JAX product offerings.
Prior to joining JAX, besides leading drug discovery efforts in oncology, he has been part of: business development teams; interacting with collaborators in industrial and academic centers, and clinical teams; co-authoring clinical investigator brochures; FDA progress reports; and clinical protocols.

Adriano Flora, Ph.D.
Director, Business Development
Opportunity Development - The Jackson Laboratory
Adriano Flora got his Ph.D. in Pharmacology and Toxicology at the University of Milano, Italy. After graduation, he moved to The Baylor College of Medicine in Houston, Texas, acquiring extensive experience using genetically modified mouse models to study the normal and pathological development of the central nervous system.
In 2011, Adriano joined the model generation branch of Taconic as Scientific Project Manager. Since 2019, he has worked as Business Development Director at The Jackson Laboratory, supporting preclinical scientists in selecting the most relevant models and services for their preclinical drug development programs.

Ralph Gareus, Ph.D.
Director, European Corporate and Commercial Development Opportunity Development - The Jackson Laboratory
Dr Ralph Gareus joined the Jackson Laboratory in September 2015 and works as Director, European Corporate and Commercial Development. After his biochemistry training at the University of Regensburg, Ralph joined the Ph.D. program of the European Molecular Biology Laboratory (EMBL) working at the Mouse Biology Unit in Monterotondo, Italy. He received his Ph.D. from the University of Heidelberg in 2002. Afterwards, he worked for Siemens Medical Solutions, focusing on in vivo molecular imaging. After additional post-doctoral training at the EMBL in Italy and the University of Cologne, Germany, Ralph joined Taconic Biosciences where he worked as scientific project manager focusing on the generation of complex genetic modifications in rodents. Ralph has more than 20 years of experience in the generation and analysis of genetically modified mice and their use in preclinical research.
Translational Humanized Models for Antibody Drug Development
Zürich Marriott Hotel
Neumuehlequai 42
8006 Zürich
14 June 2023
9:00 - Registration Open
10:15 - Opening Statements
10:20 - Immunobiology of Human Immune Engrafted Platforms for Preclinical Drug Discovery
Brian Soper Ph.D. - The Jackson Laboratory
10:45 - Application of Humanized Mouse Models in Drug Discovery
Johannes Sam Ph.D. - Roche
11:20 - The Next Generation of Humanized Mice for Translational Research
Mike Brehm Ph.D. - University of Massachusetts Chan Medical School
11:55 - Of Mice and Men - FcRn Biology and Its Implications in a Therapeutic Perspective
Simone Mester Ph.D. - Authera
12:30 - Lunch
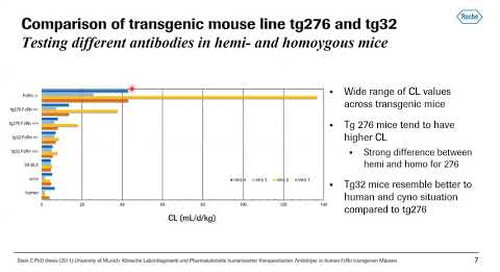
13:40 - Humanized FcRn Mouse Tg276 Model for Evaluating PK
Michael Kiffe Ph.D. - Novartis
14:15 - Humanized Mice to Test the Safety of Immunotherapies
James Keck Ph.D. - The Jackson Laboratory
14:50 - Closing Remarks
Speakers

Simone Mester, Ph.D.
CEO and Co-Founder - Authera
Simone Mester is the CEO and co-founder of Authera, a pre-clinical biotechnology company dedicated to the discovery and development of novel therapeutic biologics.
Simone has been studying antibody- and albumin-based therapeutics since 2015. In particular, her Ph.D. work focused on molecular designs with tailored pharmacokinetic properties . She was the youngest researcher to be selected to the SPARK Norway, the University of Oslo's two-year innovation program. She also won the first SPARK “pitch challenge”, awarded with a six-month stay in the incubator ShareLab in Oslo Science Park.

Michael Kiffe, Ph.D.
Senior Investigator II - DA Project Rep ATI, Research DMPK - Novartis
Michael's educational accolades include B.Sc. - M.Sc. - Ph.D. from Technical University of Braunschweig (Germany) in Organic and Natural product Chemistry, B.Sc. from University of Leipzig (Germany) in Toxicology and Environmental Care and a PostDoc with Shimadzu Corp. (Kyoto, Japan).
He has 25+ years experiences with Novartis AG Basel in different roles/positions in early drug discovery DMPK and developmental DMPK, in addition to many years of professional experiences in pharmacokinetic sciences.
He also has years of professional experiences as a project team representative in oncology and immunology in non-clinical and clinical development for all modalities (i.e. LMW, AB). Michael has worked directly with AB PK applying JAX hu-Mice models to his research.

Johannes Sam, Ph.D.
Principal Scientist in the Discovery Pharmacology Department at the Roche Innovation Center Zürich
Johannes Sam received his M.Sc. (Biotechnology) in 2009, and Ph.D. in 2014 in immuno-oncology from the Johannes Gutenberg University, Germany, and started his career as Team leader of the in vivo models group at Roche in 2014. Johannes Sam and his team have strong expertise in preclinical development and validation of therapeutic bispecific antibodies, targeted cytokines and targeted immunomodulators for cancer immunotherapy. He establishes sophisticated and translationally relevant in vivo tumor models including genetically engineered and humanized mouse models for mode of action and efficacy studies of new drug candidates. Furthermore, Johannes Sam is an experienced research project leader working from early target to clinical assessment.

Mike Brehm, Ph.D.
Associate Professor, Diabetes Center of Excellence, Program in Molecular Medicine - University of Massachusetts Chan Medical School
Dr. Brehm has over 30 years of experience in biomedical research and his laboratory is focused on the study of autoimmunity and immunological responses to infection agents and to transplantation with non-self tissues. Dr. Brehm is currently an Associate Professor in the Program in Molecular Medicine at the UMCMS and a member of the Diabetes Center of Excellence, and his laboratory is developing “humanized” mice for translational medicine and to study human T cell biology. Dr. Brehm has published over 125 manuscripts and reviews and is supported by funding from the JDRF and NIH.

Adriano Flora, Ph.D.
Director, Business Development
Opportunity Development - The Jackson Laboratory
Adriano Flora got his Ph.D. in Pharmacology and Toxicology at the University of Milano, Italy. After graduation, he moved to The Baylor College of Medicine in Houston, Texas, acquiring extensive experience using genetically modified mouse models to study the normal and pathological development of the central nervous system.
In 2011, Adriano joined the model generation branch of Taconic as Scientific Project Manager. Since 2019, he has worked as Business Development Director at The Jackson Laboratory, supporting preclinical scientists in selecting the most relevant models and services for their preclinical drug development programs.

Ralph Gareus, Ph.D.
Director, European Corporate and Commercial Development Opportunity Development - The Jackson Laboratory
Dr Ralph Gareus joined the Jackson Laboratory in September 2015 and works as Director, European Corporate and Commercial Development. After his biochemistry training at the University of Regensburg, Ralph joined the Ph.D. program of the European Molecular Biology Laboratory (EMBL) working at the Mouse Biology Unit in Monterotondo, Italy. He received his Ph.D. from the University of Heidelberg in 2002. Afterwards, he worked for Siemens Medical Solutions, focusing on in vivo molecular imaging. After additional post-doctoral training at the EMBL in Italy and the University of Cologne, Germany, Ralph joined Taconic Biosciences where he worked as scientific project manager focusing on the generation of complex genetic modifications in rodents. Ralph has more than 20 years of experience in the generation and analysis of genetically modified mice and their use in preclinical research.

Brian Soper, Ph.D.
Senior Scientific Engagement Manager - The Jackson Laboratory
Dr. Soper has worked at The Jackson Laboratory for more than 26 years. He has conducted research on hematopoietic stem cell biology, bone marrow transplantation, immune tolerance, and treatment strategies for a mouse model of human enzyme deficiency. He now works as Senior Scientific Engagement Manager providing highly technical scientific educational resources to the scientific and drug development community. Brian's expertise includes the immuno-biology of humanized mice following human PBMC or hematopoietic stem cell transplantation, and their use to study human immune modulation and immuno-oncology when co-transplanted with human tumors.

James Keck, Ph.D.
Senior Director for Innovation and Product Development - The Jackson Laboratory
Dr. Keck is the recent recipient of JAX's Presidential Innovation Fellow Award. In this role, he is responsible for developing and driving cutting-edge immuno-oncology and autoimmune platforms and services to empower preclinical drug developers across the globe, as well as overseeing internal and external collaborations that may lead to new JAX product offerings.
Prior to joining JAX, besides leading drug discovery efforts in oncology, he has been part of: business development teams; interacting with collaborators in industrial and academic centers, and clinical teams; co-authoring clinical investigator brochures; FDA progress reports; and clinical protocols.
Preclinical Evaluation of Advanced Cell-Based Therapies Using In Vivo Humanized Models
Marriott Paris Opera Ambassador Hotel
16 BD Haussmann
75009 Paris
16 June 2023
9:00 - Registration Open
10:30 - Opening Statements
10:40 - Immunobiology of Human Immune Engrafted Platforms for Preclinical Drug Discovery
Brian Soper Ph.D. - The Jackson Laboratory
11:15 - The Next Generation of Humanized Mice for Translational Research
Mike Brehm Ph.D. - University of Massachusetts Chan Medical School
12:30 - Lunch
13:30 - Molecular Shielding Protects Hematopoietic Stem Cells from Antibody-Mediated Depletion
Frank Lehmann Ph.D. - Cimeio
14:10 - Humanized Mice to Test the Safety of Immunotherapies
James Keck Ph.D. - The Jackson Laboratory
14:50 - Preclinical to Clinical Development of Next-Generation Mesenchymal Cell Therapy: From CAR-T Induced CRS in Humanized Models to Rescuing Severe COVID-19 Patients and Beyond
Tomer Bronshtein Ph.D. - Bonus Bio
15:30 - Closing Remarks
Speakers

Mike Brehm, Ph.D.
Associate Professor, Diabetes Center of Excellence, Program in Molecular Medicine - University of Massachusetts Chan Medical School
Dr. Brehm has over 30 years of experience in biomedical research and his laboratory is focused on the study of autoimmunity and immunological responses to infection agents and to transplantation with non-self tissues. Dr. Brehm is currently an Associate Professor in the Program in Molecular Medicine at the UMCMS and a member of the Diabetes Center of Excellence, and his laboratory is developing “humanized” mice for translational medicine and to study human T cell biology. Dr. Brehm has published over 125 manuscripts and reviews and is supported by funding from the JDRF and NIH.

Frank Lehmann, Ph.D.
Senior Scientist Preclinical Models - Cimeio
After studying Molecular Biotechnology, Frank did his Ph.D. at the Helmholtz Center Munich in Cancer Immunology in 2011. Thereafter, Frank worked as a Postdoc and Senior Scientist in innate immunology at the Department of Biomedicine and Children's Hospital in Basel. In 2022 Frank joined Cimeio Therapeutics AG as a Senior Scientist Preclinical Models.

Tomer Bronshtein, Ph.D.
Vice President of Business Development - Bonus Biogroup
Dr. Tomer Bronshtein is an accomplished researcher and business development expert. With over 15 years of experience in R&D, he specializes in developing advanced cell-based and cell-derived next-generation therapies. Through key research management positions, including heading the Bonus Biogroup Research Department and leading Tech Transfer at Technion's Laboratory for Cancer Drug Delivery & Cell-Based Technologies, Dr. Bronshtein has played a crucial role in the successful commercialization of innovative cell-based and cell-derived products.

Brian Soper, Ph.D.
Senior Scientific Engagement Manager - The Jackson Laboratory
Dr. Soper has worked at The Jackson Laboratory for more than 26 years. He has conducted research on hematopoietic stem cell biology, bone marrow transplantation, immune tolerance, and treatment strategies for a mouse model of human enzyme deficiency. He now works as Senior Scientific Engagement Manager providing highly technical scientific educational resources to the scientific and drug development community. Brian's expertise includes the immuno-biology of humanized mice following human PBMC or hematopoietic stem cell transplantation, and their use to study human immune modulation and immuno-oncology when co-transplanted with human tumors.

James Keck, Ph.D.
Senior Director for Innovation and Product Development - The Jackson Laboratory
Dr. Keck is the recent recipient of JAX's Presidential Innovation Fellow Award. In this role, he is responsible for developing and driving cutting-edge immuno-oncology and autoimmune platforms and services to empower preclinical drug developers across the globe, as well as overseeing internal and external collaborations that may lead to new JAX product offerings.
Prior to joining JAX, besides leading drug discovery efforts in oncology, he has been part of: business development teams; interacting with collaborators in industrial and academic centers, and clinical teams; co-authoring clinical investigator brochures; FDA progress reports; and clinical protocols.

Adriano Flora, Ph.D.
Director, Business Development
Opportunity Development - The Jackson Laboratory
Adriano Flora got his Ph.D. in Pharmacology and Toxicology at the University of Milano, Italy. After graduation, he moved to The Baylor College of Medicine in Houston, Texas, acquiring extensive experience using genetically modified mouse models to study the normal and pathological development of the central nervous system.
In 2011, Adriano joined the model generation branch of Taconic as Scientific Project Manager. Since 2019, he has worked as Business Development Director at The Jackson Laboratory, supporting preclinical scientists in selecting the most relevant models and services for their preclinical drug development programs.
Principles and Applications of Humanized In Vivo Models to Study the Immune System and Evaluate Immunotherapies
St. Pancras Renaissance Hotel
Euston Road
London, NW1 2AR
19 June 2023
9:00 - Registration Open
10:30 - Opening Statements
10:40 - Immunobiology of Human Immune Engrafted Platforms for Preclinical Drug Discovery
Brian Soper Ph.D. - The Jackson Laboratory
11:15 - The Next Generation of Humanized Mice for Translational Research
Mike Brehm Ph.D. - University of Massachusetts Chan Medical School
11:50 - The Application of Humanized Mice in Manipulation of Myeloid Cells
Ali Roghanian Ph.D. - University of Southampton
12:30 - Lunch
13:30 - Humanized Mice Supplemented with Dendritic Cells, Herpes Viruses or Lentiviral Vaccines Mount Antigen-Specific T and B Responses
Renata Stripecke Ph.D. - University of Cologne
14:10 - Humanized Mice to Test the Safety of Immunotherapies
James Keck Ph.D. - The Jackson Laboratory
14:50 - Closing Remarks
Speakers

Mike Brehm, Ph.D.
Associate Professor, Diabetes Center of Excellence, Program in Molecular Medicine - University of Massachusetts Chan Medical School
Dr. Brehm has over 30 years of experience in biomedical research and his laboratory is focused on the study of autoimmunity and immunological responses to infection agents and to transplantation with non-self tissues. Dr. Brehm is currently an Associate Professor in the Program in Molecular Medicine at the UMCMS and a member of the Diabetes Center of Excellence, and his laboratory is developing “humanized” mice for translational medicine and to study human T cell biology. Dr. Brehm has published over 125 manuscripts and reviews and is supported by funding from the JDRF and NIH.

Ali Roghanian, Ph.D.
Lecturer in Cancer Immunology & Immunotherapy -University of Southampton
Ali specialises in experimental models of cancer, as well as developing and testing the efficacy and MoA of novel biologics. Previously he was a lead scientist on a translational project, whose lead candidates (BI-1206 and BI-1607) are currently in Phase I/IIa clinical trials for the treatment of haematological and solid malignancies with promising prospects.

Renata Stripecke, Ph.D.
Professor - University Hospital of Cologne
Renata Stripecke is a full professor at the University Hospital Cologne (Germany) and directs the Institute of Translational Immuno-Oncology. Renata's focus is the development of novel gene immunotherapies for the treatment of cancer. Induced dendritic cells, CAR-T cells targeting herpes viruses and lentiviral vectors were tested preclinically in fully humanized mice. Renata is a scientific advisor for companies, reviewer for several international funding agencies and journals, and organizes periodically practical courses on humanized mice funded by JAX and EMBO.

Brian Soper, Ph.D.
Senior Scientific Engagement Manager - The Jackson Laboratory
Dr. Soper has worked at The Jackson Laboratory for more than 26 years. He has conducted research on hematopoietic stem cell biology, bone marrow transplantation, immune tolerance, and treatment strategies for a mouse model of human enzyme deficiency. He now works as Senior Scientific Engagement Manager providing highly technical scientific educational resources to the scientific and drug development community. Brian's expertise includes the immuno-biology of humanized mice following human PBMC or hematopoietic stem cell transplantation, and their use to study human immune modulation and immuno-oncology when co-transplanted with human tumors.

James Keck, Ph.D.
Senior Director for Innovation and Product Development - The Jackson Laboratory
Dr. Keck is the recent recipient of JAX's Presidential Innovation Fellow Award. In this role, he is responsible for developing and driving cutting-edge immuno-oncology and autoimmune platforms and services to empower preclinical drug developers across the globe, as well as overseeing internal and external collaborations that may lead to new JAX product offerings.
Prior to joining JAX, besides leading drug discovery efforts in oncology, he has been part of: business development teams; interacting with collaborators in industrial and academic centers, and clinical teams; co-authoring clinical investigator brochures; FDA progress reports; and clinical protocols.

Adriano Flora, Ph.D.
Director, Business Development
Opportunity Development - The Jackson Laboratory
Adriano Flora got his Ph.D. in Pharmacology and Toxicology at the University of Milano, Italy. After graduation, he moved to The Baylor College of Medicine in Houston, Texas, acquiring extensive experience using genetically modified mouse models to study the normal and pathological development of the central nervous system.
In 2011, Adriano joined the model generation branch of Taconic as Scientific Project Manager. Since 2019, he has worked as Business Development Director at The Jackson Laboratory, supporting preclinical scientists in selecting the most relevant models and services for their preclinical drug development programs.

Philip Gaskin, Ph.D.
Senior Business Development Manager - The Jackson Laboratory
Behavioural pharmacologist with a Ph.D. and industrial experience in psychopharmacology, translational neuroscience and neuropsychiatric diseases. Phil gained an Undergraduate Master's degree in Pharmacology from the University of Bath in 2009. He subsequently gained his Ph.D. from the School of Life Sciences, University of Nottingham, and has since worked in the pharmaceutical industry (Takeda, BenevolentAI) and in academia (University of Cambridge, post-doctoral research) utilizing numerous in vivo models of disease. Now he works as a Senior Business Development Manager for The Jackson Laboratory covering the UK & Ireland territory.
JAX Humanized Mice and Immunotherapy Drug Developement Resources
For the complete collection of content for our EU Symposium, please visit our resource page.
FcRn Model Platforms
This short video highlights the JAX FcRn model platform and it's use for drug discovery and development. Discover how this cutting-edge platform can evaluate drug efficacy and pharmacokinetics.
Use Of FcRn Transgenic Mice For Drug Discovery Of Therapeutic Antibodies With Roche's Michael Otteneder, Ph.D.
By evaluating IgG-based drugs in FCRN transgenic mice, researchers can gain a better understanding of how the drug will behave in humans, which can help inform dosing and administration strategies.
Evidence-Based Selection Of The Starting Dose In First-In-Human Clinical Trials Using Humanized Mouse Models
This blog discusses how humanized mice can improve drug development by reducing the risk of toxicity and adverse effects.